Introduction to Francium
Francium is one of the rarest and most unstable elements on Earth. It belongs to the alkali metal group and is highly radioactive. Due to its extreme rarity and short lifespan, it remains one of the least studied elements. Scientists are still trying to understand its unique properties and behavior. In this article, we will explore its discovery, properties, isotopes, production, applications, and challenges in detail.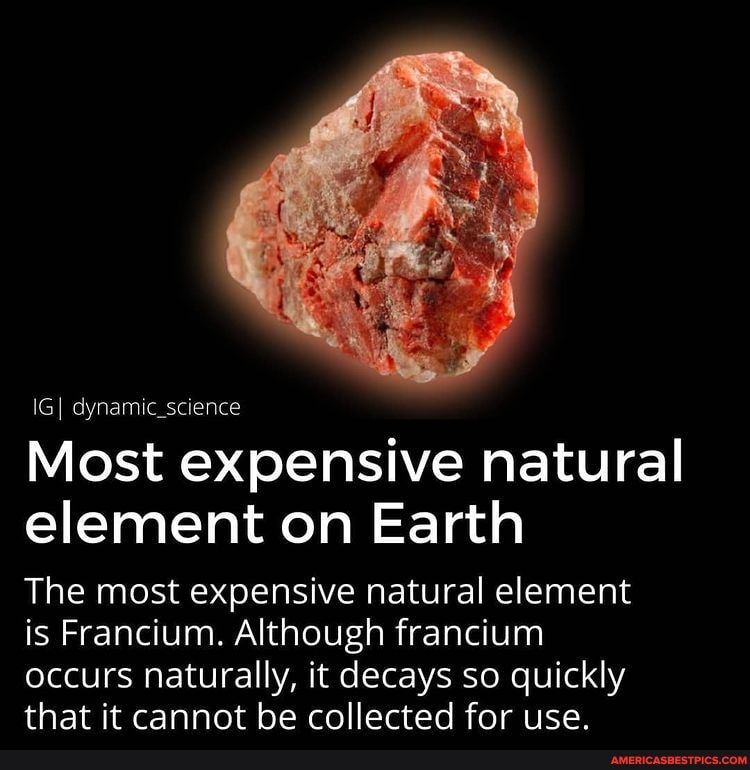
Discovery of Francium
- Discovered in 1939 by Marguerite Perey, a French physicist at the Curie Institute in Paris.

- Found while analyzing the radioactive decay of actinium.

- Initially called “eka-cesium” because of its position below cesium in the periodic table.

- Named after France to honor its scientific heritage.
- It was the last naturally occurring element to be discovered.
- Studying francium remains difficult due to its extreme instability.
Position in the Periodic Table
Francium is located in Group 1 (alkali metals) of the periodic table.:max_bytes(150000):strip_icc()/GettyImages-517345224-e456b6c739d64be89a519da75bb63e06.jpg)
- Atomic number: 87
- Chemical symbol: Fr
- Shares characteristics with other alkali metals like sodium, potassium, and cesium.
- Has a single electron in its outermost shell, making it highly reactive.
- Largest atomic radius among alkali metals.
- Lowest ionization energy, contributing to its extreme reactivity.
- Least electronegative element, meaning it easily loses electrons.
Physical and Chemical Properties
-Physical Properties

- Appearance: Believed to be silvery-white, but never observed in pure form.
- Density: Estimated at 2.48 g/cm³, though precise values remain unknown.
- Melting Point: Around 27°C (low for a metal).
- Boiling Point: Around 677°C, suggesting it easily turns into gas.
- Electrical Conductivity: Expected to be a good conductor of electricity.
-Chemical Properties
- Most reactive alkali metal, even more than cesium.
- Lowest ionization energy of all elements, making it highly unstable.
- Oxidation State: Almost always +1 due to its single valence electron.
- Explodes violently in water, similar to other alkali metals but more extreme.
- Reacts with halogens to likely form francium chloride (FrCl), francium fluoride (FrF), and francium iodide (FrI), but these have never been directly observed.
Why is Francium So Rare?
Only about 30 grams of francium exist in Earth’s crust at any time.
- Mainly produced through the decay of actinium.
- Found in uranium and thorium ores in trace amounts.
- Short half-lives make collection and study extremely difficult.
- Decays rapidly into other elements before accumulation is possible.
-Isotopes of Francium
Francium has no stable isotopes.
- Over 30 isotopes have been identified.
- Most stable isotope: Francium-223 with a half-life of 22 minutes.
- Other isotopes have half-lives from milliseconds to a few minutes.
- Decays into radium-223 or astatine-219.
- Requires advanced laboratory techniques due to rapid decay.
How is Francium Produced?
Francium cannot be extracted from natural sources in useful amounts. Instead, it is produced artificially in nuclear reactors and particle accelerators.
- Created by bombarding thorium or uranium with neutrons.
- Actinium decays to form francium as a byproduct.
- Scientists at CERN and other physics labs use high-energy collisions to study francium in controlled environments.

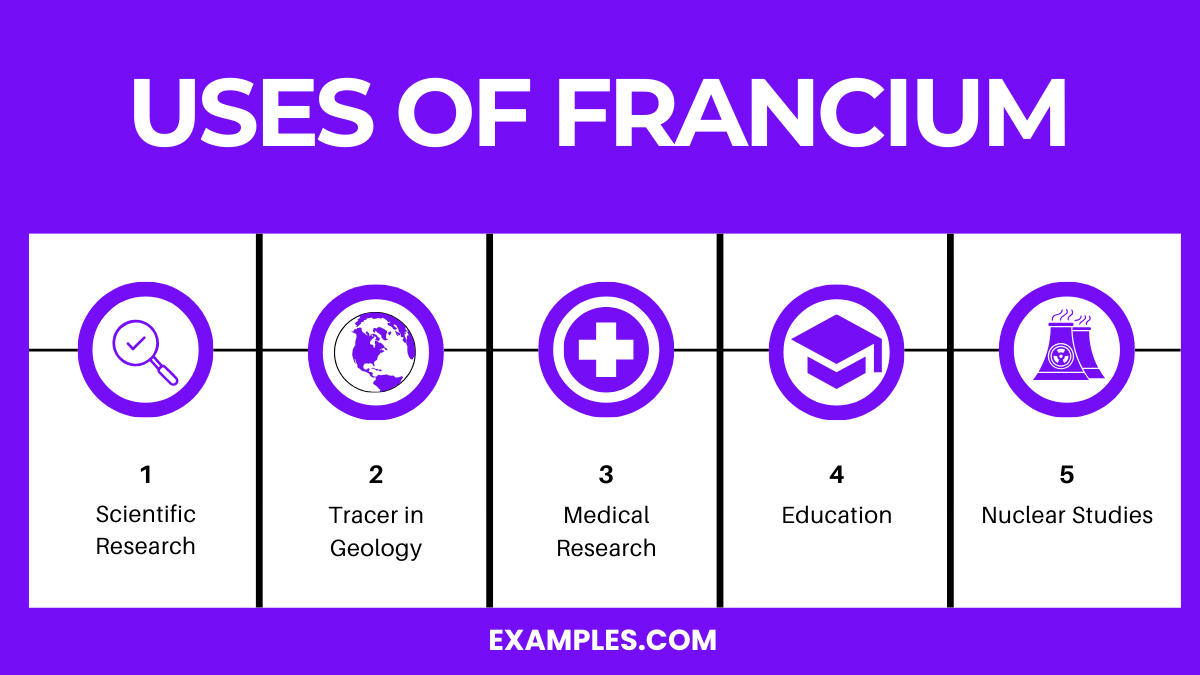
Uses of Francium
Due to its extreme rarity and instability, francium has no commercial or industrial applications. However, it is useful for scientific research.
- Used in high-precision spectroscopy to study atomic structure and quantum mechanics.
- Helps in research related to weak nuclear force interactions.
- Explored in medical research, though impractical for treatment due to radioactivity.
- Provides insights into electron behavior and fundamental physics.

The Most Reactive Metal
Francium is predicted to be the most reactive metal in the periodic table.
- Would explode violently in water, even more than cesium.
- Never directly observed due to its instability.
- Self-ignites if a large enough sample could be collected due to radiation-induced heat.
- Extremely rapid reactions with other elements due to its large atomic size and low ionization energy.
Dangers and Safety Concerns
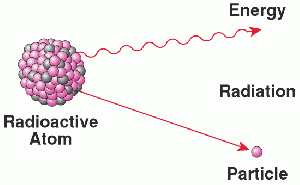
Francium is highly radioactive, making direct handling impossible.
- Emits alpha radiation, which is harmful to living tissue but does not penetrate deeply.
- Severe health risks include radiation poisoning.
- Studied only in controlled environments with strict safety protocols.
- Scientists use advanced containment and shielding to minimize radiation exposure.
- Due to short half-lives, exposure risks are lower than longer-lived radioactive elements like uranium.
Fun Facts About Francium
- Second rarest naturally occurring element (after astatine).
- A gram of francium would vaporize almost instantly due to radiation heat.
- No one has ever seen pure francium because it decays too quickly.
- The largest sample ever produced contained just a few hundred thousand atoms.
- If francium were stable, it could revolutionize chemistry due to its extreme reactivity.
- Some speculate francium could be a powerful nuclear fuel, but its instability prevents practical use.
Future Prospects and Research
Although francium is incredibly difficult to study, scientists are developing new techniques to analyze its properties.
- Laser spectroscopy techniques allow researchers to study francium without directly handling it.
- Advances in particle physics may lead to new insights into its quantum behavior.
- Understanding francium can help in refining theories related to nuclear structure and fundamental interactions.
- Future studies may unlock new applications in nuclear physics and energy research.
Conclusion
Francium is one of the most fascinating and mysterious elements in the periodic table. Despite being the most reactive and least stable alkali metal, it plays a crucial role in scientific research.
Although it has no commercial applications, its study helps scientists understand atomic structure and fundamental physics. Due to its extreme rarity and radioactivity, francium remains one of the most difficult elements to study. However, its potential for advancing scientific knowledge is undeniable.
(Click notification ![]() for more updates)
for more updates)
Artical was written by V.Harishram
''Stay true, bring facts to you''